Monosodium Glutamate (MSG) & Aspartame Environmental Neurotoxicology
Research Project INTRODUCTION
MSG is the abbreviation used for the flavor enhancer monosodium glutamate. It is used by the food industry for several reasons. The primary reason for its use is that it increases the "perceived" taste of any item that contains MSG. For instance, soup manufacturers of chicken soup have found they only need to add approximately one-half the normal amount of chicken to their product while using MSG to achieve the same taste profile as as if they added no MSG and added twice the amount of chicken. As you can see the addition of MSG to consumer products increases the profit margin of companies that use it. Unfortunately, the use of MSG appears not to be without consequence. World production of MSG in 1976 was 262,000 metric tons per year. Studies by Reif-Lehrer (67 in Special Article) reported that 25% of a population sample who have been exposed to Chinese restaurant food exhibit at least some adverse symptoms. Prior to the 1970’s, MSG was routinely added to baby food before the practice was stopped following government suggestions. Commenting on this issue, Dr. Olney, at the Department of Psychiatry, Washington University states,
In support of the NAS assumption that human infants are not affected by MSG-induced brain damage, the committee pointed to absence of behavioral manifestations in human infants given intravenous infusions of protein hydrolysates providing 0.3 g/kg/day of free glutamic acid. Dr. Olney countered by stating:
There have been at least two other MSG studies using infant primates which found no significant brain lesions after MSG exposure (Abraham et al. (10). However, in criticism of these reports, Dr. Olney states that because of the "remarkable efficiency" with which degenerate elements are removed from the scene of MSG-induced lesion (minimal lesions are cleared from mouse brain within 12 to 18 hours), it is essential to examine the brain earlier than 24 hours (Dr. Olney’s studies examined at 3 and 5 hours). This is particularly true if, due to vomiting, the infant retained very little MSG, and therefore, sustained only a minimal lesion. MSG &
Aspartate Damage Brain Hypothalamus
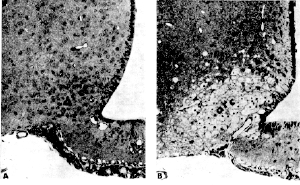
The food additives were given to a group of 75 infant mice starting at what is considered "very low" human exposure levels" of .25-.50 g/kg (grams of MSG per kilogram of animal body weight). Exposure levels continued upwards to 2 g/kg. In each case only a single dose of the compound was given. Five hours after MSG and Aspartate treatment each animal was anaesthetized and examinations were performed on the brain cells within the hypothalamus using a light microscope. The results demonstrated that only the very lowest MSG exposure level of .25 g/kg - had no observable effect upon the brain cells. However, animals receiving doses beginning at .5 g/kg of body weight (considered a normal human dose), did in fact show observable cell damage in this area of the brain. Results of all exposures are listed below: .5 g/kg dose .75 g/kg dose 1 g/kg dose 2 g/kg dose It was found that MSG and Aspartate can combine their damage potential when test animals were fed 0.5 g/kg of each compound simultaneously. In this scenario, they developed the same amount of hypothalamic damage characteristically seen in animals treated with either agent at 1 g/kg. Dr. John W. Olney Chem-Tox Comment:
Infant Seizures Improve After MSG Removal A child experiencing "innumerable seizures" at 6 months of age showed dramatic improvements after removal of MSG from the child’s diet. The case history, reported by Dr. L. Reif-Lehrer of Harvard Medical School in the journal Federal Proceedings, showed the child did not respond to dilantin treatment but had symptoms "completely alleviated by a diet that excluded exogenously added glutamate." The child’s first seizures began at 6 months of age on October 14, 1971. For the next four months the child’s seizures continued even with treatment using dilantin, mysoline and pyridoxine. At 9 months of age the child was experiencing 100 or more seizures per day. The following medical bibliography lists
chronologically
The child’s physician, Dr. M. G. Stemmermann, M.D., noted there were still no attacks as of September, 1975, except after annual test trials with an "MSG meal." Commenting on this case, Dr. Reif-Lehrer states,
Dr. Liane Reif-Lehrer, Brain Damage in Primates Evident 5 Hours After MSG Ingestion Brain lesions were found in 6 rhesus infant monkeys exposed to varying levels of a single MSG dose. Researchers at the Departments of Psychiatry and Pediatrics at the Washington University School of Medicine conducted the study with rhesus monkeys because of hypotheses from other researchers stating that susceptibility to MSG induced brain damage may be limited to sub-primates (mice, rats, rabbits, etc.) A subcommittee of nutritional experts appointed by the Food Protection Committee of the National Academy of Sciences (NAS) declared MSG to be a safe food additive requiring no regulation. The nutritional experts did admit susceptibility of infant rodents to brain damage from orally administered MSG at even the low doses of 1 gram per kilogram body weight (g/kg) but reasoned that the primate infant was most likely not at risk to MSG brain damage because it had a more mature central nervous system and more highly developed blood brain barrier at birth. However, this theory did not hold true when tested by Dr. John Olney and colleagues at the Department of Psychiatry and Pediatrics at Washington University School of Medicine. Here, the researchers exposed 6 infant rhesus monkeys to a single MSG dose ranging from 1 to 4 g/kg and were compared to 3 control monkeys exposed in the same manner to sodium salt (table salt). The researchers found that all MSG exposed monkeys developed damage to the brain area known as the infundibular region of the hypothalamus (this corresponds to the arcuate nucleus hypothalamus area in mice). Results of Dr. Olney's study:
"Our data do not support the view that only subprimates(i.e mice & rats) are susceptible to MSG-induced neurotoxicity. The lesions in our primate infants A, B and I following relatively high subcutaneous doses of MSG were so similar to those consistently observed in mice treated with high doses of MSG that it seems unrealistic to deny primate susceptibility to the MSG effect. Since lesions we detected in infants treated orally with lower doses were also similar in localization and identical in cytopathological detail to those we routinely find in infant mice treated orally with low doses of MSG, a causal link between low oral doses of MSG and necrosis (damage) of neurons in the infant primate hypothalamus also seems likely. However, since the lesions in these infants were quite small, careful consideration should be given to possible explanations other than MSG toxicity." Obesity - Shorter Growth - and Reproduction Also noted at 5 months, the MSG animals were quite lethargic as adults, and they lacked the sleekness of body coat seen in the controls. The reproductive capacity of the MSG females was also modified in that they repeatedly failed to conceive in spite of adequate exposure from 5 to 9 months of age. In summary, Dr. Olney writes, "These observations, linking MSG treatment of the neonatal mouse with a syndrome of manifestations, including skeletal stunting, marked adiposity, and sterility of the female, coupled with histopathological findings in several organs associated with endocrine function, suggest a complex endocrine disturbance. In view of the additional finding of lesions in regions of the brain thought to function as neuroendocrine regulatory centers, a unitary hypothesis might be constructed relating all or most of the findings to the neonatal disruptions of neuronal development in these centers.... The assumption that MSG is an entirely innocuous substance for human consumption has been questioned recently in view of its role in the Chines Restaurant syndrome. The finding that neuronal necrosis can be induced in the immature mouse brain by 0.5 mg/kg of MSG raises the more specific question whether there is any risk to the developing human nervous system by maternal use of MSG during pregnancy. The primate placenta maintains amino acids in consistently higher concentrations in the fetal circulation than those found in the maternal circulation, the ratio of glutamic acid being grater than 2:1. In fact, when high doses of phenylalanine were given to a pregnant rhesus monkey, the ratio of mother to fetus for this amino acid remained unchanged so that exceedingly high fetal blood levels resulted. The possibility that brain lesions could occur in the developing primate embryo in response to increased glutamic acid concentrations in the maternal circulation, therefore, warrants investigation." Dr. John W. Olney
ASPARTAME (NUTRASWEET) INTRODUCTION Aspartame is an artificial sweetener that has developed widespread use in the United States. The compound was originally given FDA approval in 1974, however, its purported safety was called into question and approval was suspended pending further investigation. In fact, final approval for consumer use was not finally given until the mid 1980’s. As with MSG, researchers Reynolds, Butler and Lemkey-Johnson (Journal of Toxicology and Environmental Health, 2: 471-480, 1976) reported that 0.5 or 1.0 g/kg of aspartame on Days 6-12 resulted in small hypothalamic lesions in neonatal mice. These results and the potentially wide use of a new artificial sweetener suggested a need for further examination of the neurobehavioral consequences of aspartame administration. Interest in and concern about aspartame has been elevated by the fact that its metabolic breakdown will liberate the amino acids phenylalanine and aspartate. BACK TO LEARNING DISABILITY INDEX
|
 There has been considerable media attention
given to the harmful health effects of MSG over the past several years. The Food
and Drug Administration (FDA) has also come out with its recent report that MSG
is "safe" for most people except for those with serious asthma difficulties.
There has been considerable media attention
given to the harmful health effects of MSG over the past several years. The Food
and Drug Administration (FDA) has also come out with its recent report that MSG
is "safe" for most people except for those with serious asthma difficulties.